Cell Therapy Manufacturing Market Analysis and Insights:
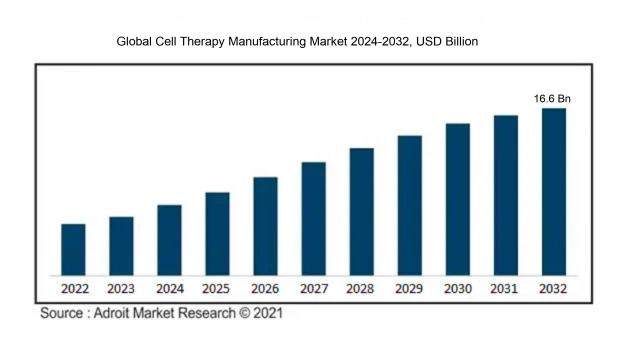
The predicted value of the worldwide cell therapy manufacturing market in 2023 was USD 5.20 billion. This market was driven by the growing number of chronic illnesses and the progress of cell therapy technology worldwide. It is anticipated that the market will grow at a compound annual growth rate (CAGR) of 15.10% from 2024 to 2032, reaching a value of USD 16.6 billion.
The market for cell therapy manufacturing is significantly influenced by various pivotal elements, notably the escalating prevalence of chronic illnesses such as cancer and autoimmune diseases, which demand cutting-edge treatment options. Innovations in cell therapy, including advancements in CAR-T cell therapy and regenerative medicine, have substantially improved treatment efficacy, drawing extensive investments towards research and development. Furthermore, the surging interest in personalized medicine is accelerating market progression, as these therapies can be specifically customized to meet the unique needs of individual patients. Favorable regulatory environments and proactive government initiatives are also playing a crucial role in market growth by simplifying approval pathways and fostering innovation. Moreover, the rise in clinical trials and partnerships among biotechnology firms, academic institutions, and research entities is promoting progress in this sector. Together, these factors are creating a vibrant landscape for cell therapy production, indicating a bright trajectory for the industry.
Cell Therapy Manufacturing Market Definition
The production of cellular therapies encompasses the methods used to generate cells intended for the treatment of a range of illnesses, such as malignancies and hereditary conditions. This process includes the growth, modification, and rigorous quality assurance of cell-based products to guarantee their safety and efficacy for clinical applications.
The manufacture of cell therapies plays a vital role in creating customized cellular products aimed at addressing multiple diseases, such as cancer, genetic anomalies, and degenerative ailments. This intricate process guarantees the safety, efficacy, and high quality of these therapies, ensuring compliance with regulatory requirements. Innovations in manufacturing methods not only increase scalability but also lower costs, thereby facilitating wider availability of cutting-edge treatments. Additionally, the expansion of regenerative medicine relies on streamlined manufacturing practices, which accelerate the introduction of new therapies and enhance patient outcomes through tailored treatment approaches. Consequently, this manufacturing framework is essential for advancing personalized medicine.
Cell Therapy Manufacturing Market Segmental Analysis:

Insights On Source
Allogenic
The global cell therapy manufacturing market is expected to be dominated by the allogenic category. This can be attributed to the increasing demand for readily available and scalable therapeutic alternatives. Allogenic therapies allow for the use of cells from a healthy donor, making them more accessible for large-scale production, which is essential for the growing patient population. Additionally, advancements in stem cell research and regenerative medicine are enhancing the viability of allogenic cell therapies. Moreover, regulatory support and ongoing clinical trials are accelerating innovation in this area, providing economic advantages and ensuring a consistent supply for patients in need.
Autologous
The market for producing cell therapies worldwide is dominated by the autologous category. By employing the patient's own cells, this method reduces the possibility of negative responses and immunological rejection. The personalized treatment aspect appeals to many patients and healthcare providers, as it increases the likelihood of successful outcomes. Despite advantages, challenges such as higher production costs and logistics involved in cell harvesting and processing may limit its growth compared to its allogenic counterpart. Nevertheless, the trend towards personalized medicine continues to sustain interest and investment in developing autologous therapies.
Insights On Indication
Cancer
Cancer is anticipated to dominate the Global Cell Therapy Manufacturing Market due to the increasing prevalence of various cancers across the globe, driving significant research and investment in innovative therapies. The rising demand for personalized medicine and advancements in technologies, such as CAR-T cell therapies, are promising outcomes for cancer treatment. Additionally, regulatory support for effective cancer therapies has encouraged numerous organizations to pursue cell-based treatments, creating a favorable environment for growth. The focus on oncology is further ened by collaborative efforts among pharmaceutical companies and research institutions, resulting in a robust pipeline of cell therapies aimed specifically at combating cancer.
HIV
HIV remains a critical area of research due to the significant global health burden it represents. There has been a continuous push towards the development of effective therapies that can not only manage the disease but also potentially offer complete cures. Increasing funding for HIV research and the advent of new technologies are propelling advancements in cell therapy solutions. However, the comprehensive global treatment approaches may limit the overall market growth as standard antiretroviral therapies become more accessible.
Autoimmune Disorders
The focus on autoimmune disorders, including diseases such as rheumatoid arthritis and lupus, has been gaining traction in the healthcare sphere. Developers are exploring cell therapies as alternative solutions to traditional treatments, which often have limited efficacy and significant side effects. Nevertheless, the complexity of autoimmune mechanisms poses challenges to therapy development, potentially slowing down market penetration compared to more straightforward applications like cancer therapies.
Immune Deficiencies
Cell therapy aimed at immune deficiencies is an emerging area with potential but still faces hurdles in widespread adoption. The market is driven by the need for innovative treatments for congenital and acquired immune deficiencies. While advancements have been made, including the use of gene therapy approaches, the pace of cell therapy development is slower than in the cancer. Hence, it does not currently dominate but is marked as an area of growth potential moving forward.
Neurological Disorders
Research into cell therapies for neurological disorders like Alzheimer's disease and Parkinson's disease is still in nascent stages. The complexity of these conditions and the nervous system's unique challenges hinder rapid advancements in treatment options. While there is significant interest in developing effective therapies, particularly with stem cell applications, the market potential has not yet reached the levels seen in oncology and other areas, making it less dominant at this time.
Insights On Manufacturing Purpose
Commercial
The commercial is expected to dominate the Global Cell Therapy Manufacturing Market due to several factors driving growth. The increasing approval of advanced cell therapies for various diseases has created a demand for large-scale production capabilities. Companies are investing significantly in scaling up manufacturing processes to meet the needs of the market and ensure product availability. Moreover, successful commercialization strategies have been established, resulting in a strong return on investment for firms focusing on this area. The shift from research-focused manufacturing to commercially viable products reflects rising patient demand, regulatory support, and technological advancements in cell therapy production methods.
Clinical
The clinical plays a crucial role in the Global Cell Therapy Manufacturing Market as it focuses on developing therapies for specific patient populations. Clinical trials are fundamental for driving innovation in cell treatments, with many organizations investing in research and development to create cutting-edge therapies. The necessity for tailored approaches that address individual patient needs is driving this, as healthcare providers seek more effective and personalized treatments. However, the clinical is primarily dependent on regulatory approvals and trial outcomes, which can introduce delays in translating discoveries to market-ready products.
Pre-clinical
The pre-clinical is essential for laying the groundwork for subsequent advancements in cell therapy. This phase involves initial laboratory research and testing that are critical for evaluating treatment safety and efficacy. The growth in this area is spurred by technological advancements and an increase in investment to expedite the development of promising therapies. While pre-clinical studies are instrumental for innovation and risk assessment, this does not generate revenue like clinical and commercial phases. Nonetheless, its importance cannot be overstated, as successful pre-clinical results significantly influence future clinical trial initiation and funding strategies.
Insights On Route of Administration
Injectable
The injectable route of administration is expected to dominate the Global Cell Therapy Manufacturing Market due to its ability to deliver therapies directly into the bloodstream or target tissues, ensuring rapid onset of action. This method is favored for critical applications, including cancers and other severe health conditions, where precision and effective delivery are crucial. The rising prevalence of such diseases and the corresponding need for advanced, efficient treatment options further bolster the growth of the injectable market. Moreover, the technological advancements in injectable formulations, including innovations in drug delivery systems, are enhancing patient compliance and overall therapeutic outcomes, reinforcing its prominence in the market.
Topical
Topical administration typically involves delivering therapies directly to the skin or mucosal membranes. This route is gaining traction due to its non-invasive nature and ease of use, particularly in dermatological applications. However, it faces limitations related to bioavailability and the penetration of complex biological materials, which may restrict the extent of its adoption in the broader cell therapy landscape. As the focus on quality and efficacy in therapy intensifies, topical routes may still remain a smaller player compared to other delivery methods.
Infusion
Infusion administration represents a technique whereby therapies are slowly delivered into the bloodstream, often used in outpatient settings for chronic conditions. While beneficial for certain therapies that require continuous dosing, infusion methods typically demand specialized equipment and healthcare settings, which can limit accessibility for some patients. Additionally, the need for longer treatment times and the potential for complications may deter healthcare providers from prioritizing infusion in cell therapy applications, keeping it subordinate to more immediate administration routes.
Implantable Bio-Scaffold
Implantable bio-scaffolds offer a unique method of delivering therapies, integrating with the patient’s own biological systems. This route is primarily used for regenerative medicine, aimed at promoting tissue healing and regeneration. Although this method shows promise, it faces challenges such as surgical intervention, risk of complications, and the need for individualized solutions, which could hinder widespread application. As such, while it presents innovative opportunities, it remains a niche in the cell therapy manufacturing landscape compared to more widely accepted methods like injectables, which offer broader adoption and applicability.
Insights On Cell Type
CAR-T Cells
The dominating area in the Global Cell Therapy Manufacturing Market is anticipated to be CAR-T Cells due to their revolutionary impact in the treatment of hematological malignancies. This sector has gained significant traction through innovative therapies that enhance the body's immune response to target and eliminate cancer cells. The commercial success of CAR-T pharmaceuticals, like Kymriah and Yescarta, demonstrates the high demand for engineered cell therapies. Investments from biopharmaceutical companies and robust research initiatives are further propelling this area as treatment options expand to solid tumors, thereby greatly driving market growth.
Hematopoietic (Blood-Forming) Stem Cells (HSC)
Hematopoietic stem cells play a vital role in regenerative medicine due to their capacity to differentiate into various blood cells. They are widely utilized in treating blood disorders, including leukemia and lymphoma, through bone marrow transplants. The well-established protocols and a strong therapeutic pipeline bolster their prominence in the cell therapy landscape. As advancements in cell processing techniques and technologies continue to progress, the efficiency in handling HSCs further boosts their adoption, ensuring they remain a cornerstone in hematologic therapies despite the rising popularity of CAR-T therapies.
Skeletal Muscle Stem Cells
Skeletal muscle stem cells are critical in the domain of muscle regeneration and repair. They hold promise in addressing muscular dystrophies and other chronic muscle diseases. The therapeutic applications involving these cells are still in their early phases but are gaining attention due to ongoing research into muscle-related disorders. Despite their potential, the challenges in isolating and expanding these cells limit their current marketability. As research continues, they may carve out a significant niche in regenerative therapies for muscular conditions.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) attract interest for their ability to differentiate into various cell types, including osteoblasts, chondrocytes, and adipocytes. They are already being explored for numerous applications in orthopedics, tissue engineering, and even immunotherapy. Their immunomodulatory properties contribute to the treatment of inflammatory and autoimmune conditions, increasing their relevance in clinical settings. Current research focuses not only on improving culture methods but also on optimizing their therapeutic efficacy, thereby paving the way for more effective applications in the regenerative medicine spectrum.
Lymphocytes
Lymphocytes, particularly T cells and B cells, are critical components of the adaptive immune system, and their manipulation underlies various immunotherapies. Their role in cell therapy is prominently noted in the design of targeted treatments for cancer, wherein they are engineered to recognize and combat specific tumor antigens. The ongoing trend towards personalized medicine further highlights the significance of lymphocytes in delivering tailored therapeutic solutions. However, the market for lymphocyte-based therapies is still emerging compared to the more established CAR-T pathways, requiring substantial investment in research and clinical trials.
Dendritic Cells
Dendritic cells serve as essential messengers in the immune response, capable of presenting antigens to T cells and stimulating their activity. Due to their pivotal role in initiating immune responses, they are being harnessed in cancer vaccines and immunotherapies. While promising, the application of dendritic cell therapies is still in a crucial developmental stage, facing challenges such as standardization and scalability in manufacturing processes. Continued research and innovative approaches to enhance their function will be instrumental in solidifying their presence in the cell therapy manufacturing market in the future.
Pancreatic Islet Cells
Pancreatic islet cells are crucial for treating conditions like Type 1 diabetes. Their therapeutic application involves transplantation to restore insulin production. However, the challenges surrounding donor shortage and immune rejection pose significant hurdles in their widespread adoption. Research is ongoing into stem cell-derived islet-like cells as a potential alternative, which may enhance future prospects. Nevertheless, current limitations hinder their immediate dominance in the cell therapy landscape, making them a more specialized with potential long-term benefits through advancements in cell technology and transplantation methods.
Insights On End User
Hospital Settings
Hospital settings are anticipated to dominate the Global Cell Therapy Manufacturing Market primarily due to their ability to provide full-spectrum care, advanced infrastructure, and access to a wide range of patient populations. The growing prevalence of chronic diseases, combined with advancements in regenerative medicine, drives hospitals to increasingly adopt cell therapy treatments. Furthermore, these institutions benefit from larger funding, which allows for the procurement of sophisticated technology necessary for cell therapy manufacturing. Hospitals also often engage in collaboration with pharmaceutical companies, further enhancing their capabilities to deliver innovative therapies efficiently. As a central part of patient care, hospitals will likely remain at the forefront of this market in the coming years.
Intensive Outpatient Treatment Centers
Intensive Outpatient Treatment Centers offer flexible care options that can cater to patients who require ongoing therapy without the need for full hospital admission. These centers play a pivotal role in the continuum of care, particularly in managing rehabilitation and recovery periods post-cell therapy. With a focus on personalized treatment plans, they are increasingly integrating cell therapy into their offerings. The ability to provide specialized services and quicker access to treatments denotes a growing importance within this space.
Academic and Research Institutes
Academic and research institutes are crucial in the development and innovation of cell therapies. They typically focus on research and clinical trials, playing a significant role in understanding the mechanisms of cell therapy. Their collaborations with biotechnology firms and healthcare providers facilitate the advancement and commercialization of new treatments, though they primarily serve as pioneers rather than primary providers. The focus on education and research makes them indispensable in shaping future cellular treatments, but their role in the manufacturing aspect is comparatively less dominant.
Specialty Clinics
Specialty clinics are recognized for delivering targeted therapies, often focusing on specific diseases, such as oncology or autoimmune disorders. While they possess expertise in particular areas, their business operations and capacity for large-scale cell therapy manufacturing usually do not match those of hospitals. Nevertheless, these clinics can play an essential role in the distribution and administration of cell therapies, as they often provide tailored treatment that addresses patient-specific needs. Their contributions are important, but they tend to be supplementary compared to the comprehensive offerings of hospital settings.
Global Cell Therapy Manufacturing Market Regional Insights:
North America
North America is poised to dominate the Global Cell Therapy Manufacturing market largely due to the prevalence of advanced healthcare infrastructure and significant investment from both private and public sectors in research and development. The presence of leading biopharmaceutical companies, innovation hubs, and a favorable regulatory environment further accelerates the growth of this market in the region. Additionally, the region boasts a well-established regulatory framework that supports clinical trials and commercialization, leading to faster market entry of new therapies. Furthermore, the increased prevalence of diseases requiring innovative treatments and the strong emphasis on personalized medicine contribute to North America's leading position in the cell therapy manufacturing sector.
Latin America
Latin America is gradually emerging in the Global Cell Therapy Manufacturing market, but it still faces challenges ahead. The region's growth is hindered by limited infrastructure and lower investment in R&D compared to more developed markets. Nevertheless, increasing awareness of cell therapies and collaborating with global pharmaceutical companies could enhance local capabilities. The growing prevalence of chronic diseases presents an opportunity for growth, yet the current market size remains relatively small.
Asia Pacific
The Asia Pacific region is witnessing rapid growth in the Global Cell Therapy Manufacturing market, driven by increasing investments in biotechnology and healthcare. Countries like China and Japan are enhancing their manufacturing capabilities to meet rising domestic and international demand. Regional governments are also implementing progressive policies that encourage innovation and clinical trials. While facing competitive pressure from North America, the Asia Pacific is establishing itself as a key player with growing market potential and a robust pipeline of new therapies.
Europe
Europe remains a significant contender in the Global Cell Therapy Manufacturing market, bolstered by strong regulatory standards and advanced R&D initiatives. The region is home to various leading biotech firms and academic institutions collaborating to enhance cell therapy techniques. Market growth is supported by increasing public and private investment in biotechnology as well as a growing patient population in need of innovative treatments. However, Europe faces stiff competition from North America, which could impact its market share in the coming years.
Middle East & Africa
The Middle East & Africa represents a nascent market in the Global Cell Therapy Manufacturing arena. Growth in this region is constrained by underdeveloped healthcare infrastructure and limited access to innovative therapies. While there is a growing interest among governments and private sectors to invest in biotechnology and improve local healthcare systems, substantial advancements remain. The emergence of clinical trials and collaborations with international companies provides potential growth avenues, but the region is not yet established as a major player in cell therapy manufacturing.
Cell Therapy Manufacturing Competitive Landscape:
The global market for cell therapy manufacturing is primarily influenced by biotechnology firms and contract manufacturing organizations, which concentrate on the development and optimization of production methods for cell-based treatments. These entities ensure adherence to regulatory requirements and address market needs. Their knowledge and innovative approaches foster progress in treatment alternatives and improve patients' access to effective therapies.
Major contributors to the Cell Therapy Manufacturing Market include Novartis AG, Gilead Sciences, Inc., Bristol-Myers Squibb Company, Amgen Inc., Miltenyi Biotec GmbH, Sartorius AG, and Lonza Group AG, among others. Additionally, Rigel Pharmaceuticals, Inc., Merck KGaA, Celltrion, Inc., Cellectis S.A., Kite Pharma (a Gilead entity), Takeda Pharmaceutical Company Limited, WuXi AppTec Co., Ltd., and R&D Systems, Inc. are also key players. These organizations are at the forefront of advancements in cell therapy, playing a vital role in the development and commercialization of cell-based therapies.
Global Cell Therapy Manufacturing COVID-19 Impact and Market Status:
The Covid-19 pandemic notably expedited the expansion of the worldwide market for cell therapy manufacturing by underscoring the demand for groundbreaking treatments and encouraging a surge in investments aimed at enhancing biomanufacturing capacities.
The COVID-19 pandemic had a profound impact on the field of cell therapy manufacturing, presenting a mix of challenges and opportunities. In the early stages, the industry faced significant disruptions, such as supply chain interruptions, workforce shortages, and postponements of crucial clinical trials, which negatively affected production and research activities. Nevertheless, the critical demand for innovative treatments during this period accelerated investments and attention on cell therapy, especially in fields such as immunotherapy and regenerative medicine.
To navigate these obstacles, companies made strategic adaptations by improving manufacturing processes, embracing automation, and enforcing rigorous safety measures to ensure continued operations. Additionally, the growing need for sophisticated therapies aimed at addressing complications related to COVID-19 fueled expansion within the sector. Consequently, while the pandemic initially presented substantial challenges, it ultimately served as a catalyst for progress in cell therapy manufacturing, encouraging partnerships between industry leaders and academic institutions, and reinforcing the importance of adaptable manufacturing solutions in the evolving healthcare landscape post-pandemic.
Latest Trends and Innovation in The Global Cell Therapy Manufacturing Market:
- In September 2023, Catalent announced the acquisition of Delphinus Medical Technologies, strengthening its capabilities in cell therapy and gene therapy manufacturing and expanding its facility footprint.
- In August 2023, Charles River Laboratories acquired Vigene Biosciences, enhancing its cell and gene therapy capabilities and offering advanced viral vector production services aimed at supporting biopharmaceutical companies.
- In June 2023, Lonza Group announced a collaboration with Moderna to expand its cell and gene therapy production capabilities in Switzerland, aiming to meet the increasing demand for mRNA and cell-based therapies.
- In May 2023, Thermo Fisher Scientific introduced a new line of cell culture media specifically designed for CAR-T cell production, aimed at improving the efficiency and scalability of manufacturing processes.
- In March 2023, WuXi AppTec revealed plans for a new state-of-the-art cell and gene therapy manufacturing facility in Pennsylvania, which is expected to provide end-to-end solutions in the field.
- In January 2023, Merck KGaA, Darmstadt, Germany, launched its new cell factory offering, BioContinuity, designed to enhance the efficiency of cell therapy development and production.
- In December 2022, Celyad Oncology announced an agreement with EdiGene to expand its CAR-T cell manufacturing capabilities in China, focusing on developing new treatment options for solid tumors.
- In November 2022, Stride Bio and Reneo Pharmaceuticals signed a strategic partnership to develop innovative gene therapies, leveraging Stride Bio's proprietary adeno-associated viral (AAV) vector technology.
Cell Therapy Manufacturing Market Growth Factors:
The expansion of the Cell Therapy Manufacturing Market is propelled by innovations in biomanufacturing techniques, a surge in research and development funding, and an escalation in the incidence of chronic illnesses.
The Cell Therapy Manufacturing Market is witnessing robust expansion due to several pivotal elements. A primary driver is the rising incidence of chronic illnesses, particularly cancer and autoimmune diseases, which has escalated the need for groundbreaking treatment options. Cell therapies are emerging as promising candidates in this regard. Additionally, advancements in bioprocessing technologies and the introduction of automated manufacturing systems have significantly enhanced production efficiency, scalability, and quality assurance, thereby facilitating adherence to regulatory requirements.
Moreover, the burgeoning field of personalized medicine has intensified interest in customized cell therapies, creating demand for sophisticated manufacturing processes capable of delivering individualized treatments. The influx of investments from both government and private entities, along with strategic partnerships between pharmaceutical firms and research institutions, is bolstering innovation and development in this area. Regulatory initiatives, such as the FDA’s expedited approval mechanisms for cell therapies, are crucial in streamlining market entry and shortening the timeline for the introduction of new products.
Furthermore, the increasing recognition of the potential therapeutic applications of stem cells and exosomes is encouraging the investigation of novel uses, thereby widening market prospects. In summary, these various factors collectively fuel the vigorous growth of the Cell Therapy Manufacturing Market, establishing it as a vital within the biopharmaceutical industry.
Cell Therapy Manufacturing Market Restaining Factors:
Critical obstacles in the cell therapy production sector encompass elevated manufacturing expenses, intricate regulatory requirements, and difficulties related to scalability and uniformity.
The growth of the Cell Therapy Manufacturing Market is hindered by several significant challenges. A primary issue is the substantial financial burden linked to the development and production of cell therapies, which limits their accessibility for both patients and healthcare providers. Additionally, the intricate regulatory landscape for these therapies often leads to prolonged approval timelines, posing risks for manufacturers looking to enter or expand within the sector. The industry also faces a shortage of skilled professionals adept in cell therapy methodologies and production techniques, which further limits its ability to meet increasing demand. Furthermore, ensuring stringent quality control is crucial, as consistency and safety in production processes can complicate operations. Supply chain disruptions, especially in acquiring essential raw materials like cell lines and growth factors, can hinder manufacturing efforts and create operational bottlenecks. Nonetheless, the landscape is shifting positively, with technological advancements and rising investments from both the private and public sectors driving innovation. This encouraging trend implies that with ongoing research and development, it is possible to effectively overcome many of these barriers to growth in the cell therapy manufacturing arena.
Key Segments of the Cell Therapy Manufacturing Market
By Source
• Autologous
• Allogenic
By Indication
• HIV
• Autoimmune Disorders
• Immune Deficiencies
• Cancer
• Neurological Disorders
By Manufacturing Purpose
• Clinical
• Commercial
• Pre-clinical
By Route of Administration
• Topical
• Injectable
• Infusion
• Implantable Bio-Scaffold
By Cell Type
• Hematopoietic (Blood-Forming) Stem Cells (HSC)
• Skeletal Muscle Stem Cells
• Mesenchymal Stem Cells
• Lymphocytes
• Dendritic Cells
• Pancreatic Islet Cells
• CAR-T Cells
By End User
• Hospital Settings
• Intensive Outpatient Treatment Centers
• Academic and Research Institutes
• Specialty Clinics
Regional Overview
North America
• US
• Canada
• Mexico
Europe
• Germany
• France
• U.K
• Rest of Europe
Asia Pacific
• China
• Japan
• India
• Rest of Asia Pacific
Middle East and Africa
• Saudi Arabia
• UAE
• Rest of Middle East and Africa
Latin America
• Brazil
• Argentina
• Rest of Latin America

